Nanobio interactions and applications
We strive for a detailed understanding of the interactions between cells and nanostructures with respect to cell behavior, cell physiology and cell mechanics. This knowledge allows us to develop novel nanoscale devices with applications in biology and medicine, such as:
- the development of nanowire devices that can interact with cells with minimal cell perturbation (e.g. for injection, nanobiopsy, mechanosensing)
- the development of nanowire tools which can control the cell behavior (e.g. with respect to differentiation, migration, proliferation, stimulation….).
Research areas:
Nanowire/nanotube interactions with membranes and cells
Our goal is to understand in detail the interactions between cells and nanostructures such as nanowires and nanotubes with respect to cell behavior, cell physiology and cell mechanics. To this aim, we use STED microscopy, phase holography microscopy, flow cytometry, immunocytochemistry and electron microscopy techniques to investigate the phenotype of a variety of cells cultured on nanowires and nanotubes. We investigate immortalized cells in terms of migration, proliferation, stress and membrane conformation on the nanowires. We also use primary retinal cells to investigate the effects of nanowires on different cell types, in particular neurons compared to glial cells. In order to deepen our understanding of the interactions between the cell membrane and nanostructures, we perform STED microscopy live imaging of the membrane of cells cultured on nanostructures. We also investigate the properties of model membranes such as supported lipid bilayer and vesicles on a variety of nanowire substrates. The accumulated knowledge is used to develop novel nanoscale devices with applications in biology and medicine.
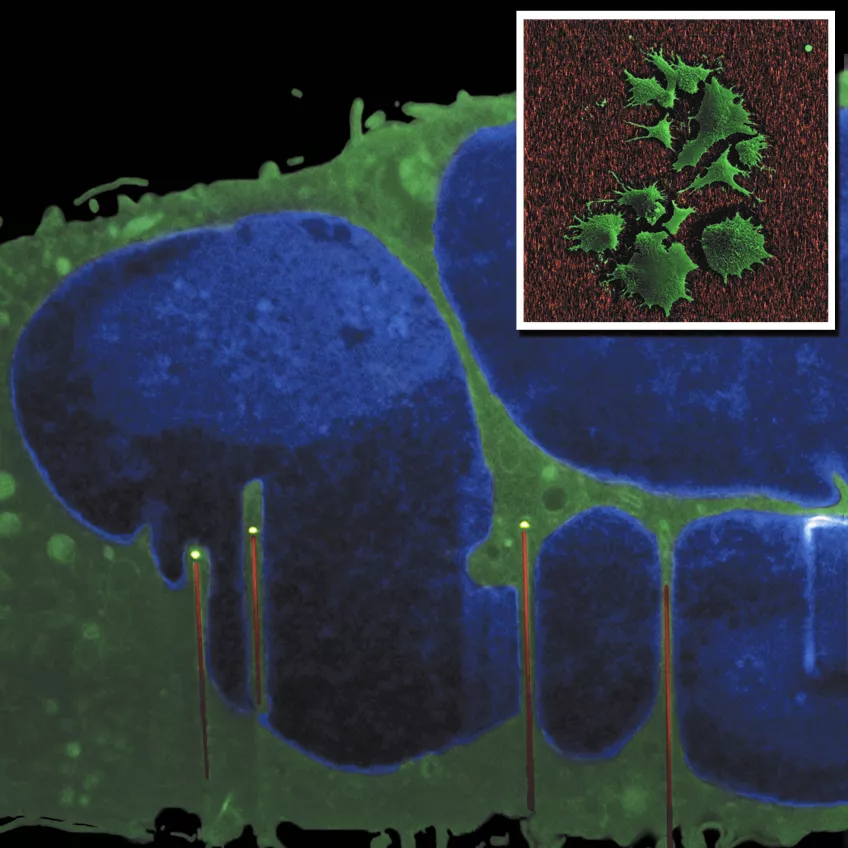
The main image shows a cross-section of a fibroblast cultured on GaP nanowires. The image was made using a FIB-SEM “slice and view” method (in collaboration with Kristian Mølhave’s group at DTU, Denmark). The cell nucleus (blue) is indented by the nanowires. Inset: SEM top view of fibroblasts on nanowires. Images by Henrik Persson (FTF) and Carsten Købler (DTU).
Cellular mechanosensing
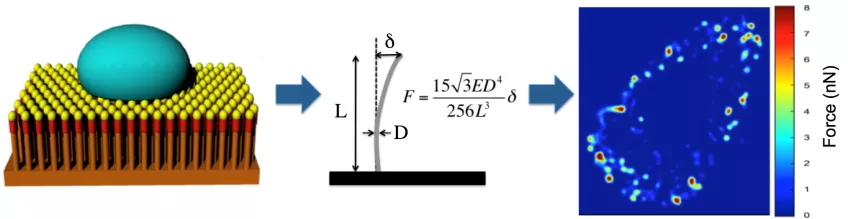
We measure cellular forces in cells and tissues with high spatial resolution using arrays of vertical nanowires. The nanowires act as cantilevers and, depending from their geometry, forces from 15 pN to 500 nN can be measured by measuring the nanowire deflection using confocal microscopy.
Measuring cellular traction forces using nanowire arrays. Cells are cultured on top of an arrays of nanowires (left). The linear elasticity theory is used to calculate the force exerted on each nanowires from the deflection of the nanowire (middle). The results can be used to create high spatial and temporal resolution force heat maps (right). Image: Zhen Li
Key publications
Single cell analysis of proliferation and movement of cancer and normal-like cells on nanowire array substrates. Z. Li, S. Kamlund, T. Ryser, S. Oredsson, C. N. Prinz. Journal of Materials Chemistry B, 6 (43), 7042-7049, 2018.
See article single cell analysis at publisher's site (new window)
Cellular Traction Forces: A Useful Parameter in Cancer Research. Z. Li, H. Persson, K. Adolfsson, L. Abariute, M. T. Borgström, D. Hessman, K. Åström, S. Oredsson, C. N. Prinz: Nanoscale, 9, 19039 –19044, 2017.
See article cellular traction forces at publisher's site
Ground State Depletion Nanoscopy Resolves Semiconductor Nanowire Barcode Segments at Room Temperature. J. Oracz, K. Adolfsson, V. Westphal, C. Radzewicz, M. T Borgstrom, S. Sahl, C. N. Prinz*, and S. W. Hell*. Nano Letters, 17 (4), 2652–2659, 2017.
See article ground state depletion at publisher's site
Electron microscopy imaging of proteins on gallium phosphide semiconductor nanowires. M. Hjort, M. Bauer, S. Gunnarsson, E. Mårsell, A. A. Zakharov, G. Karlsson, E. Sanfins, C. N. Prinz, R. Wallenberg, T. Cedervall. A. Mikkelsen. Nanoscale, 8, 3936-3943, 2016.
See article imaging of proteins at publisher's site
From immobilized cells to motile cells on a bed-of-nails: effects of vertical nanowire array density on cell behaviour. H. Persson, Z. Li, J. O. Tegenfeldt, S. Oredsson, C. N. Prinz. Scientific Reports, 5, 18535, 2015.
See article vertical nanowire array at publisher's site
Support of Neuronal Growth Over Glial Growth and Guidance of Optic Nerve Axons by Vertical Nanowire Arrays. G. Piret, M.-T. Perez, C. N. Prinz. ACS Applied Materials & Interfaces, 7 (34), 18944–18948, 2015.
See article support of neuronal growth at publisher's site
Fluid and highly curved model membranes on vertical nanowire arrays. A.P. Dabkowska, C. S. Niman, G. Piret, H. Persson, H. Wacklin, H. Linke, C. N. Prinz*, T. Nylander*. Nano Letters, 14, 4286-4292, 2014. DOI: 10.1021/nl500926y
See article model membranes on nanowire arrays at publisher's site
Fluorescent nanowire heterostructures as a versatile tool for biology applications. K. Adolfsson, H. Persson, J. Wallentin, S. Oredsson, L. Samuelson, J. Tegenfeldt, M. T. Borgström, C. N. Prinz. Nano Letters, 13(10), 4728–4732, 2013. DOI: 10.1021/nl4022754
See article nanowire heterostructures as a tool for biology at publisher's site
Fibroblasts cultured on nanowires exhibit low motility, impaired celldivision and DNA damage. H. Persson, C. Købler, K. Mølhave, L. Samuelson, J. O. Tegenfeldt, S. Oredsson, C. N. Prinz: Small, 9(23), 4006-4016, 2013. DOI: 10.1002/smll.201300644
See article fibroblasts cultured on nanowires at publisher's site
Neurite outgrowth and synaptophysin expression of postnatal CNS neurons on GaP nanowire arrays in long-term retinal cell culture. G. Piret, M-T. Perez, C. N. Prinz. Biomaterials, 34(4), 875-887, 2013. DOI: 10.1016/j.biomaterials.2012.10.042
See article neurite outgrowth at publisher's site
Fifteen-picoNewton force detection from neural growth cones using nanowire arrays. W. Hällström, M. Lexholm, D. B. Suyatin, G. Hammarin, D. Hessman, L. Samuelson, L. Montelius, M. Kanje and C. N. Prinz. Nano Letters, 10, 782-787, 2010. DOI: 10.1021/nl902675h
See article picoNewton force detection at publisher's site
Gallium phosphide nanowires as a substrate for cultured neurons. W. Hällström, T. Mårtensson, C. Prinz, P. Gustavsson, L. Montelius, L. Samuelson, M. Kanje: Nano Letters 7 (10) : 2960-2965, 2007. DOI: 10.1021/nl070728e
See article substrate for cultured neurons at publisher's site
Contact persons:
Collaborators:
- Stina Oredsson (Biology)
- Chris Madsen (Translational cancer research)
- Kalle Åström (Mathematics)
Nanobiology Spin-out

PhD4Energy
Cell-nanowire interactions for energy-related devices, funded 12 Ph.D. projects 2014-2018